Emerging Data About Combining GLP-1 Agonists with Myostatin Inhibitors
Benefits and Risks
Author
Cormac J. Mannion (Type-IIx)
Date
July 31, 2025
Introduction
GLP-1 agonists work. That's not debatable. Semaglutide (GLP-1) and tirzepatide – the incretin peptides – drop bodyweight fast, and the data supports their effectiveness for weight management. But here's the caveat: without lifting weights (resistance training), without adequate protein intake, or otherwise without some other intervention – which we'll discuss now – about 35% of that weight loss comes from lean muscle tissue, not fat. [1].
This is a problem! Despite evidence that semaglutide enhances insulin sensitivity to spare protein losses, the extreme caloric restriction that typifies severe energy restriction, such as when your appetite becomes so blunted by semaglutide that you're practically starving without noticing – or more importantly for an enhanced bodybuilder – without eating sufficient protein (and carbohydrate!) or training progressively and intensely (that only adequate carbohydrate makes possible), what inevitably happens is muscle loss. [2]. [3].
Despite the seemingly inapposite patient population for incretin peptides… the obese, the diabetic… who, quite unlike bodybuilders, are characterized by physical inactivity, to put it bluntly – there is evidence that even minor interventions like:
encouraging them to walk or get an hours' exercise a couple days per week, and
making sure they eat enough, maintaining scale weight reductions of no more than 1 lb weekly
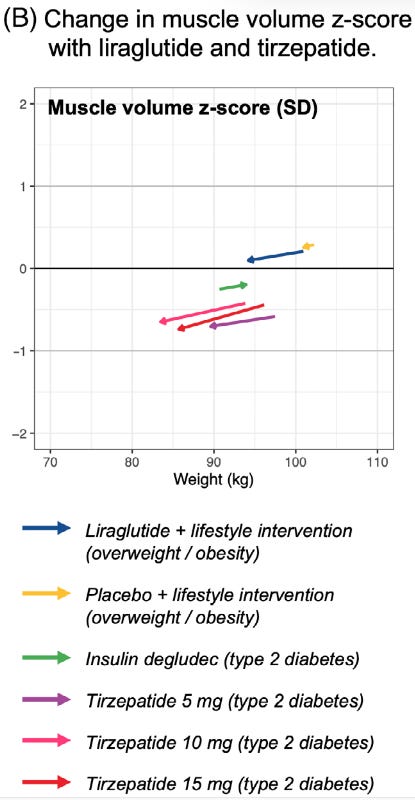
Pays real dividends by maintaining muscle as visualized here by the navy arrow:
Figure 1 – Virtually no muscle loss with simple encouragement to exercise and only lose 1 lb weekly ("lifestyle intervention")… and yes, that is good ol' insulin building muscle (green arrow). [4].
Now think – if a couple thousand steps a week, maybe some light calisthenics, the occasional pink dumbbell being lifted – and remembering to eat lunch – can make such a difference, you can safely bet the farm that a serious bodybuilder can stave off these muscle losses when he's doing everything he needs to!
So we simply cannot be complacent.
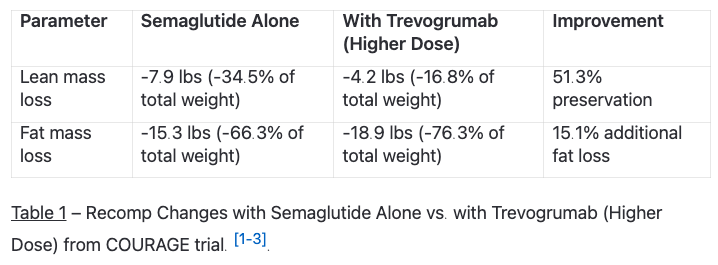
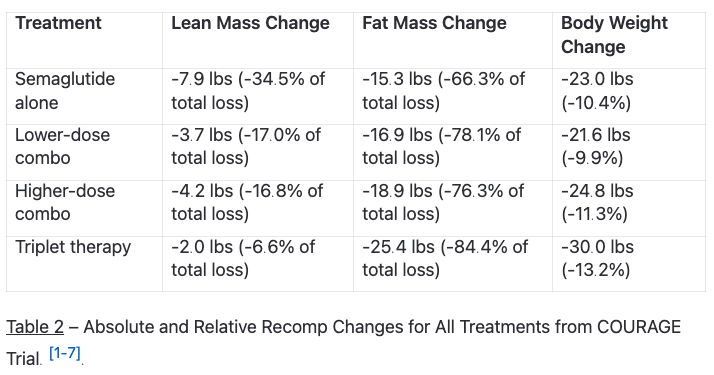
These drugs, left unchecked, can and will hinder your efforts at improving your physique. The COURAGE trial data makes this crystal clear – patients on semaglutide alone (inactive and starving) lost 7.9 pounds of lean mass out of 23 total pounds lost. [1-1].
Myostatin inhibitors offer yet another solution – indeed, an opportunity. These compounds block the body's natural brake on muscle growth, and when you combine them with GLP-1 agonists, something interesting happens: The muscle-wasting effect of severe caloric restriction arising out of the GLP-1 effects is not only neutralized in sedentary patients… but real recomp occurs – the holy grail of increased muscle mass and decreased fat mass, simultaneously. [5].
The combination creates a blend of synergistic effects (on fat loss) and a complementary effect (on muscle gain). GLP-1 agonists handle the appetite suppression and metabolic side through incretin pathways. Myostatin inhibitors protect muscle through entirely separate mechanisms involving ActRII receptors and Smad signaling. [5-1]. Different pathways, overlapping end-points (decreased fat mass, increased muscle mass).
This exploration examines the science behind this approach, focusing on the COURAGE trial findings that demonstrate substantial improvements in body composition outcomes. We'll also look at the molecular pathways that make this combination particularly effective for those seeking fat loss without muscle sacrifice.
GLP-1 Agonists: How They Work and What They Do
The molecular mechanism behind GLP-1 receptor agonists explains why these compounds have become the gold standard for obesity treatment. Unlike the old-school approaches that relied on stimulants or appetite suppressants, GLP-1 agonists work through specific hormonal pathways (i.e., the incretin GLP-1 receptor) that target both appetite regulation and metabolic function.
GLP-1 Agonists Mechanism of Action
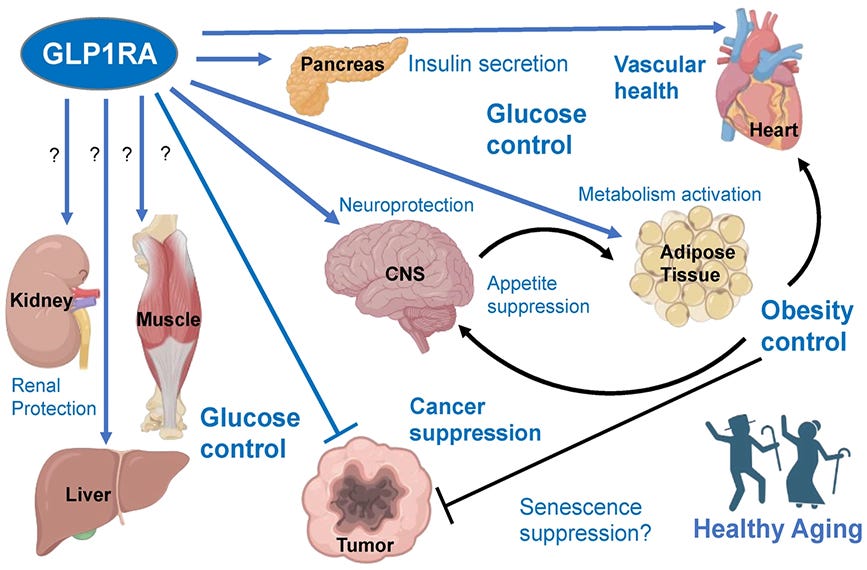
GLP-1 receptor agonists (GLP1RAs) mimic glucagon-like peptide-1, an incretin hormone naturally produced in the intestines after eating. These drugs bind to GLP-1 receptors distributed throughout the body—primarily in the pancreas, gastrointestinal tract, and brain. Once those receptors activate, they trigger multiple physiological responses that drive weight loss.
The primary mechanisms:
Gastric emptying slows down, extending satiety
Appetite suppression through direct brain action on hunger centers
Insulin secretion increases while glucagon gets suppressed
Peripheral tissue insulin sensitivity improves. [6].
These combined effects create sustained caloric intake reduction without the metabolic slowdown that typically sabotages diet-induced weight loss. Patients maintain weight reduction over extended periods compared to traditional dieting approaches.
Myostatin Inhibitors: Unlocking Muscle Growth
Belgian Blue cattle look like they're on steroids. They're not. These animals naturally lack functional myostatin – the protein that normally puts the brakes on muscle growth. The result? Extraordinary muscular development that scientists call "double-muscling." This discovery opened up an entirely new approach to muscle preservation and growth.
Myostatin Inhibitor Mechanism
Myostatin (GDF8) is part of the TGF-β superfamily, a group of cytokines that regulate cellular development and tissue homeostasis. Think of myostatin as your body's natural governor on muscle growth – it keeps things in check by actively preventing excessive muscle development.
The protein starts as a precursor that gets cleaved to become biologically active. Once mature, myostatin is expressed almost exclusively in skeletal muscle tissue, where it signals through a specific molecular cascade that's actually quite elegant in its simplicity.
Here's how it works: myostatin binds to activin receptor type II (ActRII), triggering, triggering a cascade along the intracellular signaling pathway:
- Smad2 and Smad3 proteins get phosphorylated
- These phosphorylated Smads form complexes with Smad4
- The complexes move to the nucleus
- Gene expression gets regulated, ultimately inhibiting the Akt-mTOR anabolic pathway. [5-2].
This Akt-mTOR inhibition directly suppresses muscle protein synthesis while promoting protein breakdown, the Holy Grail… and not unlike anabolic-androgenic steroids (AAS)! This is exactly why myostatin inhibitors offer a truly interesting paradigm, even though *early* drug trials were fairly underwhelming. [7].
Well, things *do* change, after all. Personally? I think the most underwhelming thing about *these* myostatin inhibitors is how hard they are to pronounce! These results bear that out. They're not AAS… but they also don't make chicks grow dicks.
But you know what the biggest difference is – and it's a huge one really – between AAS and these myostatin inhibitors are? It's a Key Takeaway for everyone, actually:
Key Takeway
Myostatin inhibitors are still under active investigation, and there's a whirlwind of activity, for treatment potential. They can treat conditions that include dysplastic disorders and muscle wasting, and here, drug-related muscle atrophy, to name but a few.
This is exciting because, unlike AAS, they haven't been relegated to the dustbin of history besides for TRT and by cowboy pill mills for the very reason that they are potentially safe!
Myostatin inhibitors work through several approaches. Follistatin, an endogenous protein regulated by β-catenin signaling, naturally antagonizes myostatin by binding directly to it. Engineered antibodies like trevogrumab work similarly – they bind to myostatin and prevent receptor interaction. Compounds like YK-11 (a SARM) operate differently by inducing follistatin expression. [8].
YK-11 is interesting in its own right, but that is a topic for another time!
Myostatin Inhibitor Benefits for Muscle Preservation
Knockout studies across species consistently show remarkable effects. Mice, cattle, and dogs with disrupted myostatin genes develop dramatically increased muscle mass through both hypertrophy (bigger fibers) and hyperplasia (more fibers). Overexpression of myostatin does the opposite – decreased muscle mass and smaller fibers.
Beyond just muscle size, myostatin inhibition offers several benefits:
Prevention of atrophy: Myostatin normally increases FoxO transcriptional activity, promoting muscle atrophy. Block myostatin, and you help preserve muscle during catabolic states. [9].
Satellite cell activation: Evidence suggests myostatin suppresses satellite cell activation and self-renewal, keeping them in quiescence. Inhibiting myostatin may enhance satellite cell activity, though some hypertrophy can occur without satellite cell involvement. [9-1].
Altered fat-muscle balance: Myostatin knockout mice show significantly decreased adipogenesis and body fat alongside increased muscle. This suggests myostatin influences pluripotent mesenchymal stem cell commitment, potentially directing cells toward fat formation rather than muscle. [5-3]. [9-2].
Clinically, several myostatin inhibitors have undergone trials – PF-06252616 (domagrozumab) by Pfizer, RO7239361 by Roche, ACE-031 and ACE-083 by Acceleron Pharma, and MYO-029 by Wyeth. Most early trials yielded suboptimal results for muscle strength improvements, highlighting the complexity of translating basic science into clinical applications. [7-1].
Myostatin Inhibitor Role in Obesity Treatment
Here's where things get interesting. Since GLP-1 agonists cause significant lean mass loss (about 35% of total weight loss), combining them with myostatin inhibitors addresses a critical limitation of GLP-1 monotherapy. [1-2].
The 2025 Phase 2 COURAGE trial demonstrated this synergistic potential. When semaglutide was combined with trevogrumab (an anti-GDF8/anti-myostatin antibody), participants experienced:
Adding garetosmab (anti-activin A) to the combination created a "triplet therapy" that preserved 80.9% of lean mass while increasing fat loss by 27.3% compared to semaglutide alone. This outcome suggests blocking both myostatin and activin A (another TGFβ superfamily member that negatively regulates muscle mass) yields superior results. [1-4]. [5-4].
Myostatin functions almost exclusively in skeletal muscle, unlike its close relative GDF11, which affects multiple organ systems. This specificity makes myostatin inhibition particularly attractive as a targeted therapy. [10].
But there are safety considerations. The triplet therapy in the COURAGE trial showed higher discontinuation rates (28.3%) compared to semaglutide alone (4.6%) or lower-dose combinations (4.1%). [1-5].
The real potential of combining GLP-1 agonists with myostatin inhibitors lies in their complementary mechanisms. GLP-1 agonists affect appetite and energy intake. Myostatin inhibitors directly target muscle maintenance pathways through separate molecular cascades. Together, they address both sides of the weight management equation: reducing fat while preserving or potentially increasing metabolically active lean tissue.
The Synergy: GLP-1 Agonists with Myostatin Blockers
Here's where it gets interesting. The COURAGE trial didn't just test these compounds separately – they combined them, and the results were pretty damn impressive.
Why This Combination Makes Sense
Look, the rationale is straightforward. GLP-1 agonists crush appetite and drop bodyweight, but they also eat your muscle. Myostatin inhibitors protect muscle through completely different pathways. Put them together and you get the best of both worlds.
The biology backs this up. GLP-1 agonists work through incretin pathways – they slow gastric emptying, suppress appetite, improve insulin sensitivity. Myostatin inhibitors work through ActRII receptors and Smad signaling. Different mechanisms, complementary effects.
Myostatin (GDF8) and Activin A both bind to activin type II receptors, triggering Smad3 phosphorylation that shuts down the Akt-mTOR anabolic pathway. That's your muscle-building pathway getting blocked. Follistatin naturally inhibits both of these proteins, which is why compounds that boost follistatin expression tend to promote muscle growth.
The combination therapy essentially mimics what follistatin does naturally – it removes the brakes on muscle growth while GLP-1 agonists handle the fat loss side.
What the COURAGE Trial Actually Showed
The 2025 Phase 2 COURAGE trial was designed specifically to test this approach. They divided treatment into two 26-week periods and compared semaglutide alone against various combinations with muscle-preserving antibodies. [1-6].
The interim analysis confirmed what we suspected – semaglutide alone resulted in 34.5% of weight loss coming from lean tissue. But here's what happened when they added myostatin inhibition:
The Body Composition Data
The COURAGE trial data shows exactly what happens when you combine these mechanisms:
The triplet therapy – adding garetosmab (anti-activin A) to semaglutide and trevogrumab – preserved 80.9% of lean mass while increasing fat loss by 27.3% compared to semaglutide alone. That's not just preservation of muscle; that's actually enhanced fat loss.
Preserving lean mass during weight loss isn't just about aesthetics. It maintains metabolic rate, prevents adaptive thermogenesis, preserves functional capacity, and reduces sarcopenia risk. Plus, muscle mass improves insulin sensitivity and glycemic control – factors that work synergistically with GLP-1 therapy.
Practical Implications
This isn't just academic. The combination creates a fundamentally different metabolic state than equivalent weight loss from GLP-1 monotherapy alone.
At the molecular level, you're getting complementary effects on Smad signaling pathways. Myostatin normally activates Smad2/3 signaling, which inhibits muscle growth. BMPs activate Smad1/5/8 pathways that promote muscle hypertrophy. By blocking myostatin and Activin A, the combination shifts this balance toward anabolism even during caloric restriction.
The safety profile needs consideration though. Lower-dose combinations showed similar tolerability to semaglutide alone, but triplet therapy had higher discontinuation rates (28.3% vs. 4.6% for semaglutide alone). The effectiveness comes with trade-offs that need careful dose optimization.
These findings suggest we're looking at a real shift in obesity treatment – from simple weight reduction to targeted body composition improvement through synergistic biological pathways.
Safety Profile
Safety data from the 26-week analysis revealed important considerations for clinical implementation. [1-8]. The combination of semaglutide with trevogrumab alone showed a safety profile comparable to semaglutide monotherapy:
Treatment-emergent adverse events (TEAEs): 64.9% (semaglutide) vs. 68.2% (combinations)
Treatment discontinuation rates: 4.6% (semaglutide) vs. 4.1% (lower-dose combo) and 10.6% (higher-dose combo)
The triplet therapy exhibited more tolerability challenges, with 28.3% of patients discontinuing treatment and higher rates of severe TEAEs (10.1% vs. 2.0% with semaglutide alone). [1-9]. This suggests careful dose optimization and individual consideration is needed for the triplet approach.
Clinical Implications
The COURAGE trial findings reshape the approach to obesity management by demonstrating that:
Quality of weight loss matters as much as quantity
Targeted biological interventions can specifically preserve muscle while enhancing fat loss
Multi-mechanism approaches yield superior body composition outcomes
The trial establishes a clear scientific principle that blocking GDF8 with or without activin A can preserve muscle and increase fat loss in patients treated with GLP-1 therapy. As noted by George D. Yancopoulos, Chief Scientific Officer at Regeneron, these findings are consistent with preclinical data in rodents and non-human primates, suggesting a robust biological effect. [1-10].
After the weight-loss phase, patients entered a weight-maintenance phase receiving either higher-dose trevogrumab monotherapy or placebo through week 52. Data from this second phase will provide critical insights into whether myostatin inhibition alone can maintain the improved body composition achieved during initial treatment.
Conclusion
The COURAGE trial data changes everything. We now have proof that the muscle loss problem with GLP-1 agonists isn't something you just have to accept. Myostatin inhibitors fix it, and they do it while making fat loss even better.
This isn't just an incremental improvement – it's a complete shift in how we should think about weight loss. Scale weight means nothing if you're losing the wrong tissue. Body composition is what matters, and this combination delivers on that front.
The molecular pathways make sense. Smad signaling, follistatin regulation, β-catenin activity – these aren't just academic concepts. They're the mechanisms that explain why blocking myostatin while using GLP-1 therapy works so damn well for preserving muscle during fat loss.
Future applications will refine the dosing. The triplet therapy showed higher discontinuation rates, but that's a dosing issue, not a fundamental problem with the approach. Body composition should be the primary metric for evaluating any weight management intervention – not just dropping pounds.
Bolus
A Practical and Reference Guide for the Use of Human Growth Hormone and GH Secretagogues
For those interested in growth hormone and related pathways, my book, Bolus, covers the deeper science behind these anabolic mechanisms.
The safety profile needs optimization, sure. But the core principle is solid: you can specifically target fat while protecting muscle. That's been the holy grail of body composition improvement, and this combination delivers it through well-understood biological pathways.
GLP-1 agonists paired with myostatin inhibitors represent the future of intelligent weight management. The days of accepting muscle loss as collateral damage for fat loss should be over.
Key Takeaways
Understanding the hidden truth about GLP-1 agonist benefits reveals critical insights for optimizing weight management through strategic combination therapy.
• GLP-1 agonists cause significant muscle loss: Approximately 35% of weight loss from semaglutide comes from lean tissue, not fat, creating a major limitation for body composition goals.
• Myostatin inhibitors preserve muscle during weight loss: Blocking myostatin with trevogrumab preserved 51% of lean mass that would otherwise be lost with GLP-1 therapy alone.
• Combination therapy enhances fat loss while protecting muscle: The COURAGE trial showed triplet therapy increased fat loss by 27% while preserving 81% of lean mass compared to semaglutide alone.
• Molecular synergy drives superior outcomes: GLP-1 agonists work through appetite pathways while myostatin inhibitors target Smad signaling, creating complementary effects on body composition.
• Safety profile requires careful consideration: While dual therapy showed similar tolerability to monotherapy, triplet combinations had higher discontinuation rates (28% vs 5%).
The revolutionary potential of this combination lies in shifting obesity treatment from simple weight reduction to targeted tissue-specific improvements, fundamentally changing how we approach body composition optimization in clinical practice.
About the Author
Type-IIx is an expert on all methods used in enhanced bodybuilding and the author of Bolus: A Practical and Reference Guide for the Use of Human Growth Hormone and GH Secretagogues. His articles can be found on Meso-Rx, his Ampouletude website, and here on Substack, along with his other projects like the Gear, Growth, and Gains Podcast on the web [www] – [telegram] – and everywhere podcasts stream!
For services and products, visit Type-IIx’s LinkTree
References
“Interim Results from Ongoing Phase 2 COURAGE Trial Confirm Potential to Improve the Quality of Semaglutide (Glp-1 Receptor Agonist)-Induced Weight Loss by Preserving Lean Mass | Regeneron Pharmaceuticals Inc.” Regeneron – Press Releases, https://newsroom.regeneron.com/news-releases/news-release-details/interim-results-ongoing-phase-2-courage-trial-confirm-potential/. Accessed 16 Jul. 2025.
(Type-IIx), Cormac Mannion. “Semaglutide and Tirzepatide Are More Than Just Weight Loss Drugs.” MESO-Rx, Nov. 2023, https://thinksteroids.com/articles/ozempic-mounjaro-weight-loss-glp1-gip-agonists-partitioning-agents/.
Volpe, Sara, et al. “Once-Weekly Semaglutide Induces an Early Improvement in Body Composition in Patients with Type 2 Diabetes: A 26-Week Prospective Real-Life Study.” Nutrients, vol. 14, no. 12, Jun. 2022, p. 2414. PubMed, https://doi.org/10.3390/nu14122414.
Neeland, Ian J., et al. “Changes in Lean Body Mass with Glucagon-Like Peptide-1-Based Therapies and Mitigation Strategies.” Diabetes, Obesity and Metabolism, vol. 26 Suppl 4, Sep. 2024, pp. 16–27. PubMed, https://doi.org/10.1111/dom.15728.↩︎
Mastaitis, Jason W., et al. “GDF8 and Activin A Blockade Protects Against GLP-1–Induced Muscle Loss While Enhancing Fat Loss in Obese Male Mice and Non-Human Primates.” Nature Communications, vol. 16, no. 1, May 2025, p. 4377. www.nature.com, https://doi.org/10.1038/s41467-025-59485-9.
American Society of Health-System Pharmacists: ASHP_. American Society of Health-System Pharmacists, 2014, p. 3217-8. http://www.ashp.org/
Bond, Peter. “Where Are the Myostatin Inhibitors?” PeterBond.Org, 15 Dec. 2020, https://peterbond.org/post/where-are-the-myostatin-inhibitors.
Kanno, Yuichiro, et al. “Selective Androgen Receptor Modulator, YK11, Regulates Myogenic Differentiation of C2C12 Myoblasts by Follistatin Expression.” Biological & Pharmaceutical Bulletin, vol. 36, no. 9, 2013, pp. 1460–65. PubMed, https://doi.org/10.1248/bpb.b13-00231.
Dubois, Vanessa, et al. “Androgens and Skeletal Muscle: Cellular and Molecular Action Mechanisms Underlying the Anabolic Actions.” Cellular and Molecular Life Sciences: CMLS, vol. 69, no. 10, May 2012, pp. 1651–67. PubMed, https://doi.org/10.1007/s00018-011-0883-3.
Suh, Joonho, and Yun-Sil Lee. “Similar Sequences but Dissimilar Biological Functions of GDF11 and Myostatin.” Experimental & Molecular Medicine, vol. 52, no. 10, Oct. 2020, pp. 1673–93. PubMed, https://doi.org/10.1038/s12276-020-00516-4.




This is exactly the kind of content, for why I signed up for a one-year membership yesterday, right away.
Very few people in the bodybuilding industry are as academic as you… you don’t spout bro science, you teach/share in a very academic manner, and that’s why im here 💪🙏